
Diagnostic Holders
Cartridge and strip holders for lateral flow and ELISA platforms with tight fit, smooth edges, and consistent opacity.
Precision holders, cases, and accessories engineered for reliability. Validated processes, controlled environment, and rigorous product release for regulated healthcare markets.
We manufacture single‑use plastic components for diagnostics, point‑of‑care devices, and hospital workflows. Our teams partner with medical device brands to industrialize designs, ensuring robust quality and on‑time delivery.
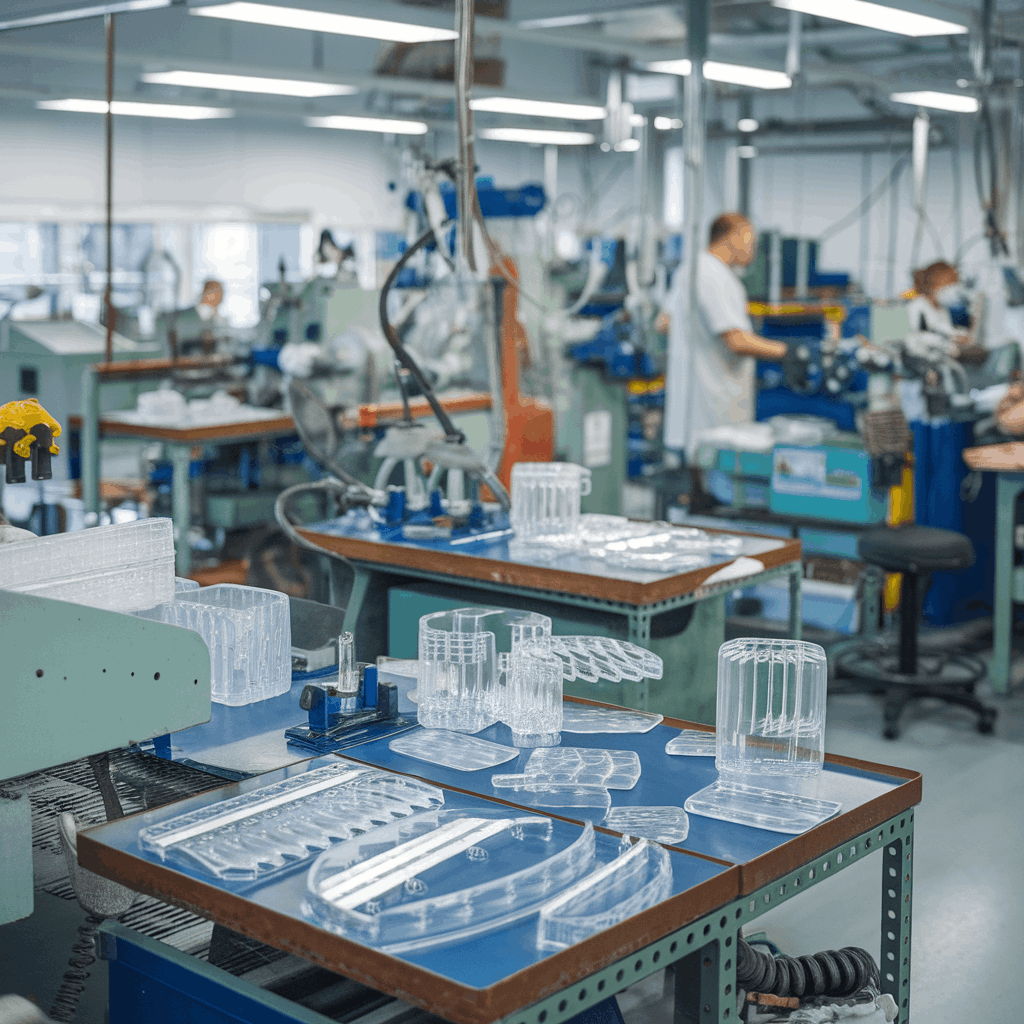
High‑consistency disposable plastic parts for medical use cases.

Cartridge and strip holders for lateral flow and ELISA platforms with tight fit, smooth edges, and consistent opacity.

Protective cases and trays for single‑use instruments, designed for sealing and labeling operations.

Caps, clips, and protective covers to stabilize and secure medical components during use and transport.

Partner network for precision tools and quick‑turn mold iterations with documented validations.

Vision checks, gauge studies, and SPC dashboards ensure capability and conformance across lots.

Light assembly, pouching, kitting, and labeling to streamline downstream sterilization and shipping.
Our system reflects ISO 13485 principles with documented procedures, recorded training, and continuous risk‑based improvements.
Regulatory alignment with Malaysia’s Medical Device Authority (MDA). Learn more.
“Repres Jebu delivered consistent quality across our first three lots and supported fast engineering changes without missing delivery windows.” — APAC Diagnostics Lead
We operate as an extension of your quality system, providing full traceability, robust documentation, and responsive customer service from Kuala Lumpur to your global facilities.
We actively reduce waste and energy intensity while maintaining compliance and product safety.

Repres Jebu Medical Plastics Sdn. Bhd.
Lot 8, Jalan Sungai Besi, 57100 Kuala Lumpur, Malaysia
Phone: +60 3-9212 4587
Email: contact@represjebu.org